Biomarker Testing
Your Superpower starts with 100+ biomarkers.
Here is everything we test.
Testing is performed by CLIA-certified, CAP-accredited reference laboratories. Results support clinician interpretation and risk assessment and are not intended as stand-alone diagnoses or treatments. Superpower reports a mix of direct and derived biomarker metrics.
What Is Biomarker Testing?
At Superpower, biomarker testing is the foundation of everything we do. It’s not about guesswork, quick fixes, or “one-size-fits-all” health advice. Biomarker testing provides precise, objective data about biological processes and disease risk by measuring specific molecules in blood samples, allowing for a more personalized and proactive approach to health compared to generic advice or symptom-driven care.
Why It Matters
Waiting for symptoms to appear can mean missing the opportunity for early intervention, as many conditions remain silent until they are advanced. Biomarker testing can improve patient care by enabling:
- Detection of diseases such as some cancers, cardiovascular conditions, and diabetes, increasing the chance for intervention and potential outcomes, though the accuracy and impact may vary by disease and biomarker used.
- Understanding individual health baselines to optimize strategies for energy, metabolism, and longevity, particularly by tracking biomarkers related to these functions over time.
- Monitoring health changes in response to new diet, exercise, or supplement regimens, helping to objectively measure the impact of lifestyle adjustments.
- Personalizing health decisions, including risk assessment and treatment options, rather than relying on average population data or standard guidelines alone.
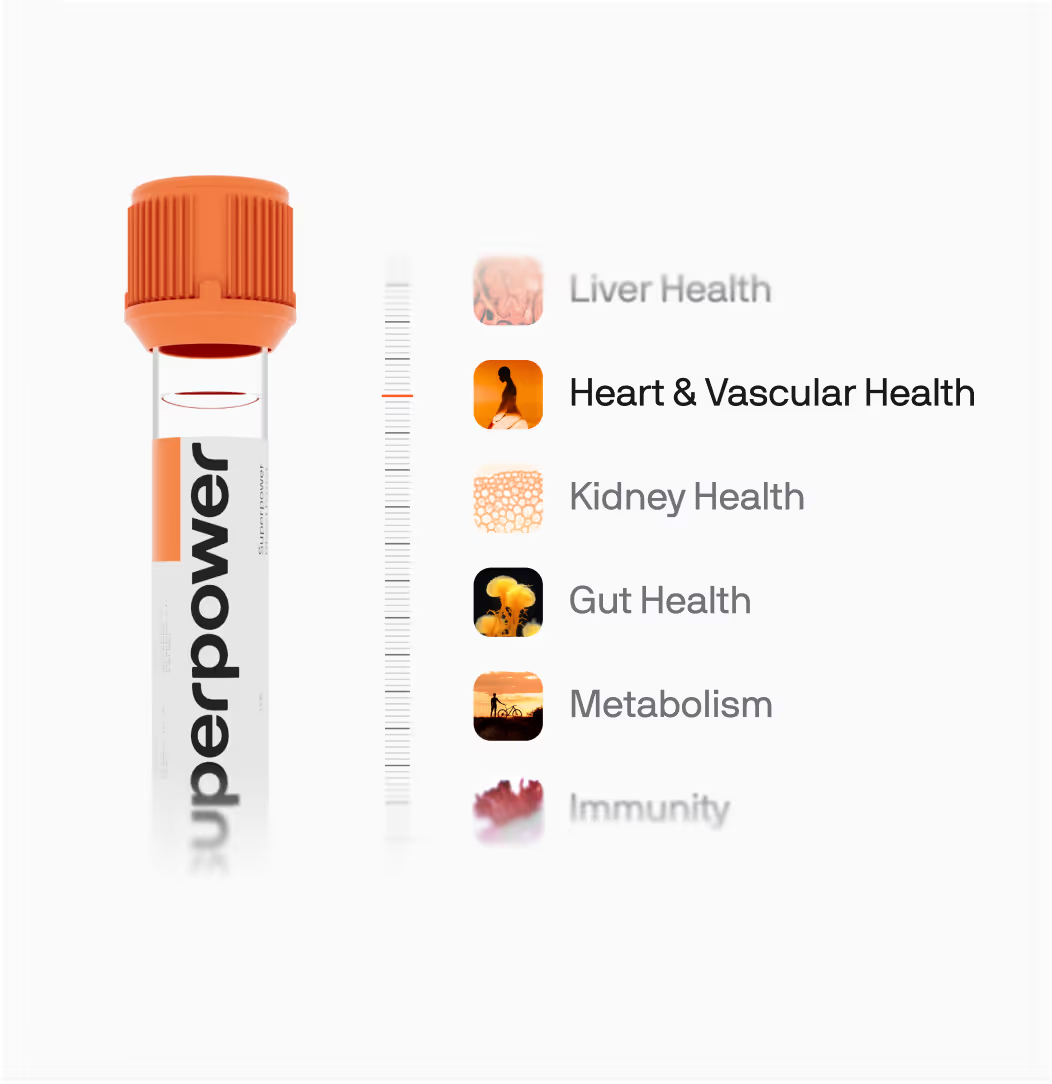
Biomarkers We Test At Superpower
Heart & Vascular Health Biomarkers
All cholesterol except good cholesterol; includes LDL, VLDL, and other atherogenic particles that contribute to plaque buildup and cardiovascular disease.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Good cholesterol that helps remove bad cholesterol from arteries; higher levels are protective against heart disease.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A type of fat in blood; high levels increase risk of heart disease and pancreatitis.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Bad cholesterol that can build up in arteries; high levels increase risk of heart disease and stroke.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The Castelli Risk Index I reflects the balance between all circulating cholesterol and protective HDL; lower values indicate healthier lipid profiles and reduced cardiovascular risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
The Castelli Risk Index II compares bad cholesterol to good cholesterol; provides targeted insight into the balance between atherogenic and protective lipoproteins.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
The total amount of cholesterol in blood; high levels increase risk of heart disease.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A protein that carries bad cholesterol; elevated levels increase risk of cardiovascular disease.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A type of lipoprotein associated with an increased risk of cardiovascular diseases.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The total number of LDL particles in circulation; a stronger predictor of cardiovascular disease than LDL cholesterol, as each particle can contribute to arterial plaque formation.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
Integrates immune activation and lipid protection; elevated ratios are strongly associated with cardiovascular events, mortality, and systemic inflammation.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: neutrophils, HDL-C.
A powerful marker of insulin resistance and metabolic syndrome; elevated ratios suggest metabolic dysfunction and increased cardiovascular risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: TG, HDL-C.
Calculated as the logarithm of triglycerides to HDL ratio; reflects lipid quality and predicts cardiovascular risk compared with individual lipid markers.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: TG, HDL-C.
The concentration of small, dense LDL particles that are highly atherogenic; these particles penetrate arterial walls more easily and are strongly linked to cardiovascular risk.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
The average diameter of HDL particles; larger HDL particles are generally more effective at cholesterol removal and cardiovascular protection than smaller particles.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
The concentration of large, mature HDL particles that help remove cholesterol from arterial walls and provide anti-inflammatory protection.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
The average diameter of LDL particles; larger particles are less atherogenic than smaller, denser particles that more readily penetrate arterial walls and promote plaque formation.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
The concentration of large, triglyceride-rich VLDL particles; elevated levels indicate poor lipid metabolism and are associated with insulin resistance and metabolic dysfunction.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
Shows the ratio of potentially harmful cholesterol to protective HDL; higher values indicate increased risk of plaque formation and inflammation.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: Total-C, HDL-C.
Expresses the proportion of total cholesterol made up of LDL; higher ratios indicate greater cardiovascular risk as more cholesterol is in atherogenic particles.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: LCL-C, Total Cholesterol.
The total number of HDL particles that perform reverse cholesterol transport; a stronger predictor of cardiovascular protection than HDL cholesterol, reflecting functional capacity.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
The average diameter of VLDL particles; larger particles indicate triglyceride overload and inefficient fat metabolism, often linked to insulin resistance and liver dysfunction.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
Reflects cholesterol content per LDL particle and serves as a marker of particle size; low ratios indicate small, dense, more atherogenic LDL particles.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: LDL-C, ApoB.
Compares uric acid levels to protective HDL cholesterol; elevated ratios may indicate increased metabolic and cardiovascular risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: uric acid, HDL-C.
Provides insight into lipoprotein particle size and composition; low ratios suggest small, dense LDL particles that are more atherogenic.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: TG, ApoB.
Reflects cholesterol content per atherogenic particle; low ratios suggest cholesterol-poor, small dense particles linked to increased cardiovascular risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: Non-HDL-C, ApoB.
Liver Health Biomarkers
An enzyme found in liver and bones; elevated levels may indicate liver disease or bone disorders.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Compares albumin to globulin proteins; helps assess liver function, protein metabolism, and immune status.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: albumin, globulin.
The main protein in blood that maintains fluid balance; low levels may indicate liver disease, kidney disease, or malnutrition.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A waste product from red blood cell breakdown; elevated levels may indicate liver disease or blood disorders.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Blood proteins including antibodies; abnormal levels may indicate immune disorders, liver disease, or infections.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
An enzyme found in liver and muscles; elevated levels may indicate liver damage or muscle injury.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A liver enzyme sensitive to alcohol and bile duct problems; elevated levels may indicate liver disease.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The processed form of bilirubin; elevated levels may indicate liver disease or bile duct problems.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The unprocessed form of bilirubin; elevated levels may indicate blood disorders or liver problems.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Calculated: total - direct.
Measures the proportion of total cholesterol carried by potentially harmful lipoproteins; higher ratios indicate increased atherosclerotic risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: Non-HDL-C, Total-C.
Reflects the balance between oxidative stress and lipid protection; elevated ratios suggest liver stress and increased cardiometabolic risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
Compares unconjugated to conjugated bilirubin; helps differentiate between hemolytic disorders and liver dysfunction.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: indirect bilirubin, direct bilirubin.
Assesses the relationship between bilirubin levels and albumin; useful for evaluating liver synthetic function and bilirubin metabolism.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: total bilirubin, albumin.
Kidney Health Biomarkers
Reflects the relationship between urea and creatinine waste products; helps distinguish between dehydration, blood loss, kidney dysfunction, or liver issues.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
Essential mineral for bones, muscles, and nerves; abnormal levels may indicate bone disease, kidney problems, or hormonal disorders.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
An electrolyte essential for heart and muscle function; abnormal levels can cause dangerous heart rhythm problems.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Measures bicarbonate levels in blood; helps assess acid-base balance and kidney function.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A waste product filtered by kidneys; elevated levels indicate decreased kidney function.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
An electrolyte that helps maintain fluid balance; abnormal levels may indicate kidney problems or dehydration.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
An electrolyte that helps maintain fluid balance; abnormal levels may indicate kidney problems or dehydration.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Estimates how well kidneys filter waste; lower values indicate decreased kidney function.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
A waste product filtered by kidneys; elevated levels indicate decreased kidney function.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Sex Hormones Biomarkers
A protein that binds sex hormones; affects the amount of active hormones available to tissues.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Testosterone that is available for use by tissues; includes free and loosely bound testosterone.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
A hormone precursor that declines with age; low levels may affect energy, mood, and immune function.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The active form of testosterone not bound to proteins; more accurately reflects hormone activity.
Method: Laboratory-developed test (LDT) validated under CLIA; not cleared or approved by the FDA. Results are interpreted by clinicians in context and are not a stand-alone diagnosis.
A hormone that regulates reproductive function; levels help assess fertility and menopause status.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The unbound form of PSA; helps distinguish between benign and malignant prostate conditions.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A hormone important for menstrual cycle and pregnancy; levels help assess reproductive health.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A protein produced by the prostate; elevated levels may indicate prostate problems including cancer.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A hormone that triggers ovulation and testosterone production; helps assess reproductive health.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A hormone that stimulates milk production; abnormal levels may affect fertility and sexual function.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Estimates biologically active testosterone by comparing total testosterone to SHBG; useful for assessing androgen status in both men and women.
Method: Derived from laboratory results. If any input is measured by a laboratory-developed test (LDT) validated under CLIA, that input is not cleared or approved by the FDA. This ratio/index itself is not FDA-cleared. Results support clinician interpretation and are not a stand-alone diagnosis. Inputs: total testosterone, SHBG.
Reflects hormonal balance between androgens and estrogens; imbalances are linked to cardiovascular risk, inflammation, and metabolic dysfunction.
Method: Derived from laboratory results. If any input is measured by a laboratory-developed test (LDT) validated under CLIA, that input is not cleared or approved by the FDA. This ratio/index itself is not FDA-cleared. Results support clinician interpretation and are not a stand-alone diagnosis. Inputs: testosterone, estradiol.
Metabolic Health Biomarkers
Converts HbA1c into estimated average blood glucose over 2-3 months in mmol/L units; reflects long-term glycemic control and metabolic health.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
Converts HbA1c into estimated average blood glucose over 2-3 months; provides an intuitive measure of long-term glucose control in familiar glucose meter units.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
A waste product that can form crystals in joints; high levels may cause gout or kidney stones.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A hormone that regulates blood sugar; elevated levels may indicate insulin resistance or diabetes risk.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Adjusts total calcium based on albumin levels to estimate biologically active calcium; accounts for protein binding effects on calcium measurement.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: total calcium, albumin.
Estimates insulin resistance using fasting triglycerides and glucose; higher values suggest metabolic dysfunction and increased diabetes risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: TG, glucose.
Nutrients Biomarkers
The protein in red blood cells that carries oxygen; low levels indicate anemia while high levels may suggest dehydration or lung conditions.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The average amount of hemoglobin in each red blood cell; useful for diagnosing different types of anemia.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The percentage of blood volume made up of red blood cells; helps assess for anemia, dehydration, or blood disorders.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Cells that carry oxygen throughout your body; low levels may indicate anemia while high levels may suggest dehydration or lung disease.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The average size of red blood cells; helps classify different types of anemia and nutritional deficiencies.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The concentration of hemoglobin in red blood cells; helps identify specific types of anemia and blood disorders.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Blood cells responsible for clotting; low levels increase bleeding risk while high levels may increase clotting risk.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Measures variation in red blood cell size; elevated levels may indicate nutritional deficiencies or blood disorders.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The average size of platelets; helps assess platelet function and bone marrow activity.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The total amount of proteins in blood; abnormal levels may indicate liver disease, kidney disease, or nutritional problems.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The storage form of vitamin D; low levels may cause bone problems, muscle weakness, and immune dysfunction.
Method: Usually performed by FDA-cleared immunoassay in CLIA-certified, CAP-accredited laboratories. If an LC/MS method is used at the performing site, the assay is a laboratory-developed test (LDT) validated under CLIA and not cleared or approved by the FDA. Results support clinician interpretation and are not a stand-alone diagnosis.
Compares red cell size variation to average cell size; helps characterize different types of anemia and red blood cell disorders.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: RDW, MCV.
Inflammation Biomarkers
A marker of inflammation; elevated levels increase risk of heart disease and other inflammatory conditions.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Measures how quickly red blood cells settle; elevated levels indicate inflammation or infection.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Composite marker using platelets, neutrophils, and lymphocytes; reflects immune imbalance and systemic inflammation linked to cardiovascular risk.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: CBC differentials.
Compares iron storage protein to albumin; helps assess nutritional status and inflammatory burden in complex clinical scenarios.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: ferritin, albumin.
Balances immune activation against lipid protection; elevated ratios indicate increased inflammation and reduced cardiovascular protection.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: monocytes, HDL-C.
Compares inflammatory marker to nutritional protein; elevated ratios indicate systemic inflammation with potential nutritional compromise.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: CRP, albumin.
Reflects inflammatory and thrombotic status; elevated ratios may indicate increased cardiovascular risk and systemic inflammation.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: platelets, lymphocytes.
Composite inflammatory marker using neutrophils, monocytes, and lymphocytes; indicates systemic inflammatory burden.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: neutrophils, monocytes, lymphocytes.
Thyroid Health Biomarkers
Estimates free thyroid hormone levels; helps assess thyroid function more accurately.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
A molecule that acts like a messenger, telling your thyroid gland how much hormone to produce; abnormal levels can signal an underactive or overactive thyroid.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The main thyroid hormone; abnormal levels indicate overactive or underactive thyroid.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The active form of thyroid hormone; more accurately reflects thyroid function than total T3.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Energy Biomarkers
A protein that stores iron; low levels indicate iron deficiency while high levels may indicate iron overload or inflammation.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The stress hormone; abnormal levels may indicate adrenal disorders or chronic stress.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Measures the blood's capacity to bind iron; helps diagnose iron deficiency or overload.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The percentage of iron-binding sites that are occupied; helps assess iron status and storage.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis.
Essential mineral for oxygen transport; low levels cause anemia while high levels may indicate iron overload.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Immune System Biomarkers
Immune system cells that help fight infections and diseases; abnormal levels may indicate infection, immune disorders, or blood cancers.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The actual number of eosinophils in your blood; useful for diagnosing allergic conditions and parasitic infections.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The actual number of lymphocytes in your blood; important for evaluating immune system health.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
White blood cells that fight viral infections and produce antibodies; levels help assess immune system function.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The actual number of basophils in your blood; helps evaluate allergic reactions and certain blood conditions.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The actual number of monocytes in your blood; helps assess immune response and inflammatory conditions.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
White blood cells that fight bacterial infections; elevated levels often indicate bacterial infection or inflammation.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
White blood cells that fight infections and remove dead cells; elevated levels may indicate chronic infection or inflammation.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
White blood cells that fight parasites and are involved in allergic reactions; elevated levels may indicate allergies or parasitic infections.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
White blood cells involved in allergic reactions and inflammation; elevated levels may indicate allergic conditions or blood disorders.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The actual number of neutrophils in your blood; helps assess immune function and infection risk.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Reflects immune balance and inflammatory status; lower ratios may indicate chronic inflammation or immune dysfunction.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: lymphocytes, monocytes.
Compares platelet count to total white blood cells; provides insight into hematologic balance and potential inflammatory states.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: platelets, WBC.
A marker of systemic inflammation and immune stress; elevated ratios are associated with increased cardiovascular risk and mortality.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: neutrophils, lymphocytes.
Reflects balance between innate and adaptive immunity; elevated ratios may indicate chronic inflammatory conditions.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: monocytes, lymphocytes.
Advanced inflammatory marker combining multiple immune cell types; reflects complex immune-inflammatory interactions.
Method: Derived from FDA-cleared laboratory results. This ratio/index is not an FDA-cleared test. It aids clinician-directed risk assessment and monitoring and is not a stand-alone diagnosis. Inputs: neutrophils, lymphocytes, platelets.
Detects antibodies highly specific to lupus; rising levels often indicate increased disease activity, especially in the kidneys.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Note: For Quest members, dsDNA is not included. In NY/NJ, the ANA panel includes dsDNA, SSA-52, SSA-60, SS-B, Smith, and RNP antibodies.
Screens for autoimmune activity often associated with lupus and related connective tissue diseases.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Note: For Quest members, a positive ANA will automatically trigger follow-up testing of the titre and pattern. For NY/NJ members, the ANA panel includes dsDNA, SSA-52, SSA-60, SS-B, Smith, and RNP antibodies.
Screens for antibodies that indicate celiac-related autoimmunity and gluten-triggered immune activity.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
Note: This panel is designed as a screening test. It does not include additional markers found in a full comprehensive panel
Body Composition Biomarkers
DNA Health Biomarkers
Essential vitamin for nerve function and red blood cell formation; deficiency causes anemia and neurological problems.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
An amino acid that can damage blood vessels; elevated levels increase risk of heart disease and stroke.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
A B vitamin essential for DNA synthesis and red blood cell formation; deficiency causes anemia and birth defects.
Method: FDA-cleared clinical laboratory assay performed in CLIA-certified, CAP-accredited laboratories. Used to aid clinician-directed evaluation and monitoring. Not a stand-alone diagnosis.
The following biomarkers Progesterone, Vitamin K, ADMA/SDMA, IGF-1, Thyroid Peroxidase, Lipoprotein Fractionation, NMR and Thyroglobulin Antibody are not currently offered for Superpower members residing in New York/New Jersey.
Some methods are laboratory-developed tests (LDTs) validated under CLIA but not cleared or approved by the U.S. FDA. Public claims mirror the performing laboratory’s intended-use language. Clinicians interpret results in context and may order confirmatory testing where appropriate.
Plus add-on diagnostic testing available
Access comprehensive tests well beyond mainstream healthcare limits.

Custom blood panels

Gut microbiome

Cancer screening
Developed by world-class medical professionals
Supported by the world’s top longevity clinicians and MDs.




Dr Anant Vinjamoori
Superpower Chief Longevity Officer, Harvard MD & MBA

Dr Leigh Erin Connealy
Clinician & Founder of The Centre for New Medicine

Dr Abe Malkin
Founder & Medical Director of Concierge MD

Dr Robert Lufkin
UCLA Medical Professor, NYT Bestselling Author

membership
$17

.avif)
.avif)
Superpower
Membership
.avif)
Your membership includes:
- Annual full body testing across 100+ biomarkers
- A custom action plan built on your biology and goals
- 17 health scores and your biological age
- Al Chat to dig deeper into your data
Many concierge clinics charge $10k – $100k for their services, we’ve built technology to make the world’s best healthcare as accessible as possible.
You will be able to schedule a 15 minute appointment (blocked out just for you) at one of our partner clinics. At home visits can also be scheduled for an additional fee.
- Understand your results in a beautiful dashboard
- 24/7 message access to a concierge care team, with answers within 24 hours on weekdays
- Lab draw at-home option (extra charge)
- Only one draw needed rather than two thanks to our partnership with Quest
- Discounted access to our supplement marketplace. Highly curated brands at big savings for the lifetime of your membership
- Personalized action plan
- AI chat with all of your data
No insurance needed. One flat fee, no co-pays or surprise charges. HSA/FSA cards accepted.
Superpower specializes in prevention-based testing and treatments and is not intended for emergency or immediate health issues.
While you will have a Superpower concierge, your annual membership is designed to complement a primary care doctor if you have one, not replace them.
We are happy to help you share any test results with an outside provider to ensure you receive well-rounded medical care.
Most primary care doctors aren’t trained to run this kind of advanced testing. We’ve negotiated special lab rates so we can offer 100+ tests at a fraction of the usual cost — often 1/4th the price.
The Superpower Baseline Panel is already comprehensive.
It covers 10 times more biomarkers than a typical annual physical.
The Advanced Panel is designed to go deeper, with three scenarios in particular.
- Uncovering risks that don’t show up in standard labs
Markers like Lp(a), LDL lipoprotein fractionation help identify cardiovascular risk even when cholesterol looks normal.
- Know what drives energy, metabolism and hormones
Tests like insulin, IGF-1, thyroid antibodies, and inflammatory markers explain symptoms that basic hormone tests can’t.
- Reveal deeper patterns
Inflammation, autoimmunity, and nutrient markers (e.g. homocysteine, ESR, thyroid antibodies) can show what’s driving long-term health trends.
Individuals with family history of heart disease, unresolved symptoms, or a desire to understand inflammation and hormone health especially benefit from testing.







Frequently Asked Questions
Read moreBiomarker testing is a laboratory analysis of genes, proteins, hormones, or other molecules in your blood or tissues to help assess your health, identify risk factors, or guide personalized care decisions. To learn more, read our guides on biomarker testing.
Biomarker testing can identify hidden health risks, nutritional deficiencies, hormone levels, or early signs of disease — allowing for targeted lifestyle or medical interventions before symptoms develop. To learn more, read our guides on specific illnesses and diseases that biomarker testing can assist with.
People interested in optimizing health, longevity, or athletic performance, men and women monitoring hormone status (including testosterone), or those with specific health concerns (like heart, thyroid, or metabolic health) all benefit from biomarker testing.
Our biomarker tests at Superpower require a simple blood draw — either at home or in a clinic. Some other tests use additional samples like urine or saliva, depending on what is being measured.
Yes, certain biomarkers are linked to risks for cardiovascular disease, diabetes, hormone imbalances, metabolic syndrome, and even some cancers, enabling early and personalized prevention strategies.
For most people, testing every 6–12 months is recommended for ongoing monitoring and tracking changes in health or the effectiveness of interventions, though frequency can vary based on goals and medical advice.

“Best health check of my entire life.”
Vinay Hiremath, Founder of Loom

“Life changing”
Jordi Hayes, Founder of Capital.xyz
It is our belief that if you improve your health, you can improve every other aspect of your life.
However, mainstream medicine has not helped many of us do that.
It is often one size fits all, reacts too late, and misses the full picture.
We built Superpower to change that.






.svg)



.png)