Cancer screening wasn’t designed to look everywhere. It was designed to look for specific cancers, at specific ages, in specific populations.
That model has saved lives. But it also means most screening is still gated by age or risk factors: You turn 55. You smoke. You have a family history of breast cancer.
With this approach, many cancers are only discovered after symptoms appear, and patients are left playing catch-up, resorting to scorched-earth treatments like chemotherapy and radiation.
When cancer is identified early - before it spreads - treatment options expand and five-year survival rates can be up to four times higher than when diagnosed at later stages.¹
Still, our system still depends on waiting for the right trigger to begin testing. With nearly one in two Americans expected to face cancer in their lifetime, this is an ineffective way to manage a disease.

The Limits of Current Screening
Here’s the gap: Even though 40% of people develop cancer in their lifetime, only ~14% of cancers in the U.S. are detected through recommended screening.2,3
Most are still found only after symptoms emerge, when disease is often more advanced and harder to treat.
At the same time, cancer incidence in younger adults continues to rise, but screening hasn’t kept pace. It was designed for a world where cancer was rare in younger people and slow to emerge. That is no longer the world we live in.
This is where new technology begins to matter.
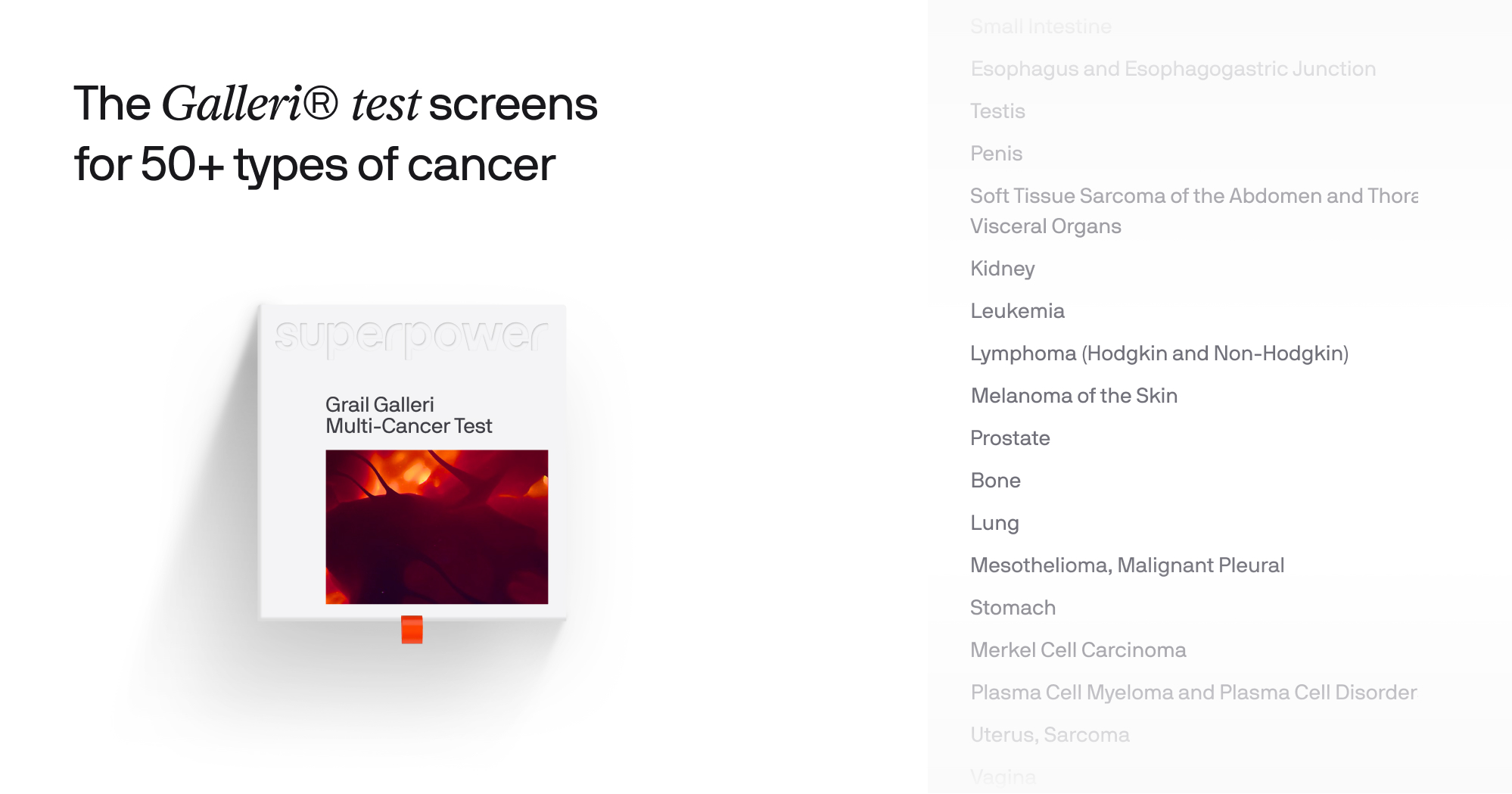
The Galleri® Test: A Different Model of Screening
The Galleri® multi-cancer early detection test from GRAIL represents a fundamentally different approach to cancer screening.
Instead of looking at one organ at a time, the test analyzes fragments of cell-free DNA that tumors shed into the bloodstream. These fragments carry distinct epigenetic patterns - specifically DNA methylation signals -that differ between healthy and cancer cells.
If a cancer signal is detected, the test can also predict where in the body the cancer is most likely coming from.
Unlike single biomarkers or organ-specific tests, methylation patterns are:
- Present across many cancer types
- Detectable at early stages
- Highly specific to cancer biology
Rather than screening one cancer at a time, the test can screen for a signal associated with 50+ cancer types, including many with no routine screening today, such as pancreatic, ovarian, liver, and certain blood cancers.
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. Galleri is a screening test and does not diagnose cancer. Diagnostic testing is needed to confirm cancer. The Galleri test should be used in addition to healthcare provider recommended screening tests.
Don't Leave Your Health to Chance
Cancer survivors often speak about “luck”: I’m so lucky I caught it early, I’m lucky they found it, I’m one of the lucky ones.
But with a disease this prevalent, outcomes shouldn’t be so heavily influenced by chance and timing.
We can’t control everything about cancer. But we can improve how we look for it. We can strengthen our screening strategies so they’re designed to surface signals earlier, rather than waiting for symptoms to demand action. And we can expand what we screen for.
Nearly 70% of cancer-related deaths are caused by cancers with no recommended screening .4 For many of these cancers, there's no standard age to begin testing, no annual reminder, no established protocol. That reality reflects the limits of the technology that was available, and a reactive system built around those constraints.
But those constraints are changing.
Advances in blood-based multi-cancer early detection now allow us to look more broadly across cancer types.Tests like Galleri surface information earlier, mitigate surprise, and provide more options and time for decisions.
That’s why we offer Galleri: to expand access to advanced testing you wouldn't typically get in standard care, and give you more control in your health.
*The Galleri test has not been shown to reduce deaths due to cancer or improve overall survival.
Nearly 70% of cancer-related deaths are caused by cancers with no recommended screening .4 For many of these cancers, there is no age when screening automatically begins, no annual reminder, no standard protocol. The system waits for symptoms.
New advances in blood-based screening are beginning to challenge that model. By identifying cancer signals in the bloodstream before symptoms appear, tests like Galleri surface information earlier, when there may be more treatment pathways and more time to make decisions.
Important Safety Information
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those age 50 or older. The test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. The Galleri test is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of the test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs, and symptoms. A test result of No Cancer Signal Detected does not rule out cancer. A test result of Cancer Signal Detected requires confirmatory diagnostic evaluation by medically established procedures (e.g., imaging) to confirm cancer. If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False positive (a cancer signal detected when cancer is not present) and false negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
Laboratory/Test Information
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed — and its performance characteristics were determined — by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high- complexity testing. The Galleri test is intended for clinical purposes.
References
- Surveillance, Epidemiology, and End Results Program. (2026). Cancer survival statistics by stage at diagnosis. National Cancer Institute. https://seer.cancer.gov/statistics/types/survival.html
- National Cancer Institute. (2025). Cancer statistics: Understanding lifetime risk (SEER Program). U.S. Department of Health and Human Services. https://www.cancer.gov/about-cancer/understanding/statistics
- NORC at the University of Chicago. (2022). State-specific cancer detection statistics: Charts 1–13. https://www.norc.org/content/dam/norc-org/pdfs/State-Specific%20PCDSs%20chart%201213.pdf
- Nature Medicine. (2025). Challenges in early cancer detection. Nature. https://www.nature.com/articles/d41586-025-00530-4



.svg)







.png)
.png)



.avif)